UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
(Exact name of registrant as specified in its charter)
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
| |
||
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code:
N/A
(Former name or former address, if changed since last report.)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered | ||
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 8.01 | Other Events. |
On December 5, 2020, Kura Oncology, Inc. (the “Company”) announced preliminary results from its KOMET-001 Phase 1/2A clinical trial that were presented at an oral presentation at the 2020 Annual Meeting of the American Society of Hematology (“ASH”). As of the data cutoff date for the ASH presentation, November 2, 2020, the trial had enrolled 12 patients with relapsed or refractory acute myeloid leukemia (“AML”), of whom ten were evaluable for safety and tolerability and eight were evaluable for efficacy. Clinical or biological activity was reported in six of the eight efficacy-evaluable patients, including two patients achieving a complete remission, one patient achieving a morphological leukemia-free state, and one patient experiencing a marked decrease in hydroxyurea requirements and having attained peripheral blood count stabilization. As presented at ASH, KO-539 has been well tolerated with a manageable safety profile to date. As of the data cutoff date, no drug discontinuations due to treatment-related adverse events and no evidence of QTc prolongation or other clinically significant EKG changes were reported. Treatment related adverse effects (grade ³ 3) were reported to included pancreatitis, increased lipase, decreased neutrophil count, tumor lysis syndrome and deep venous thrombosis.
On December 5, 2020, representatives of the Company began providing presentation materials (the “Presentation”) to certain interested parties. A copy of the Presentation is attached hereto as Exhibit 99.1 and incorporated herein by reference.
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits.
| Exhibit |
Description | |
| 99.1 | Presentation Materials of Kura Oncology, Inc. | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Date: December 7, 2020 | Kura Oncology, Inc. | |||||
| By: | /s/ James Basta | |||||
| James Basta | ||||||
| Chief Legal Officer and Secretary | ||||||

DEVELOPING PRECISION MEDICINES FOR THE TREATMENT OF CANCER Corporate Presentation – December 2020 Exhibit 99.1

This presentation contains forward-looking statements. Such statements include, but are not limited to, statements regarding our research, preclinical and clinical development activities, plans and projected timelines for tipifarnib and KO-539, plans regarding regulatory filings, our expectations regarding the relative benefits of our product candidates versus competitive therapies, and our expectations regarding the therapeutic and commercial potential of our product candidates. The words “believe,” “may,” “will,” “estimate,” “promise,” “plan”, “continue,” “anticipate,” “intend,” “expect,” “potential” and similar expressions (including the negative thereof), are intended to identify forward-looking statements. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Risks that contribute to the uncertain nature of the forward-looking statements include: our preclinical studies and clinical trials may not be successful; the U.S. Food and Drug Administration (FDA) may not agree with our interpretation of the data from clinical trials of our product candidates; we may decide, or the FDA may require us, to conduct additional clinical trials or to modify our ongoing clinical trials; we may experience delays in the commencement, enrollment, completion or analysis of clinical testing for our product candidates, or significant issues regarding the adequacy of our clinical trial designs or the execution of our clinical trials may arise, which could result in increased costs and delays, or limit our ability to obtain regulatory approval; the commencement, enrollment and completion of clinical trials and the reporting of data therefrom; the COVID-19 pandemic may disrupt our business and that of the third parties on which we depend, including delaying or otherwise disrupting our clinical trials and preclinical studies, manufacturing and supply chain, or impairing employee productivity; our product candidates may not receive regulatory approval or be successfully commercialized; unexpected adverse side effects or inadequate therapeutic efficacy of our product candidates could delay or prevent regulatory approval or commercialization; and we may not be able to obtain additional financing. Additional risks and uncertainties may emerge from time to time, and it is not possible for Kura’s management to predict all risk factors and uncertainties. All forward-looking statements contained in this presentation speak only as of the date on which they were made. Other risks and uncertainties affecting us are described more fully in our filings with the Securities and Exchange Commission. We undertake no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made. Forward-Looking Statements

*Cash, cash equivalents and short-term investments as of September 30, 2020 Targeted Oncology Advancing two wholly owned, targeted oncology drug candidates using a precision medicine approach; fast-to-market strategy Proprietary Pipeline Tipifarnib: Farnesyl transferase inhibitor Registration-directed trial in HRAS mutant head and neck squamous cell carcinoma (HNSCC) ongoing Opportunity to expand to HRAS and PI3Kα dependent tumors Multiple clinical proof-of-concept studies support significant lifecycle expansion opportunities KO-539: Menin inhibitor Potent and selective inhibitor of the menin-KMT2A(MLL) protein-protein interaction Potential to target ~35% of acute myeloid leukemia (AML) Preliminary Phase 1 data show encouraging safety, tolerability and clinical activity in multiple genetically defined subgroups of AML Strong Financials $325.4 million in cash* provides runway into 2023 Investment Highlights

Proven oncology drug development and commercialization expertise Kura Leadership Team and Board of Directors Leadership Team Troy Wilson, Ph.D., J.D. President & Chief Executive Officer James Basta, J.D. Chief Legal Officer Stephen Dale, M.D. Chief Medical Officer Kirsten Flowers Chief Commercial Officer Kathleen Ford Chief Operating Officer Marc Grasso, M.D. Chief Financial Officer & Chief Business Officer Board of Directors Faheem Hasnain Executive Chairman, Gossamer Bio Robert Hoffman Former Chief Financial Officer, Heron Therapeutics Thomas Malley President, Mossrock Capital Diane Parks Former Head of U.S. Commercial, Kite Pharma Steven Stein, M.D. Chief Medical Officer, Incyte Mary Szela President and CEO, TriSalus Life Sciences Troy Wilson, Ph.D., J.D. President and CEO, Kura Oncology

Advancing Targeted Oncology Drug Candidates Using a Precision Medicine Approach Targeting KMT2A(MLL)-r and NPM1-Mutant AML Orphan Drug Designation Opportunity to address large patient population with high unmet need in relapsed/refractory AML Publications support potential to drive robust and persistent responses in KMT2A(MLL)-r and NPM1-mutant AML Targeting HRAS Mutant Solid Tumors Fast Track Designation Initial opportunity to address high unmet need in relapsed/refractory HRAS mutant HNSCC Opportunities to expand to broader patient populations and to additional indications KO-539 Tipifarnib

TIPIFARNIB IN HRAS MUTANT SOLID TUMORS

Tipifarnib in HRAS Mutant Solid Tumors Unique MOA targets farnesylation, an essential modification required for activity of the HRAS mutant oncoprotein Phase 2 data demonstrates treatment response of ~ 50% ORR, ~ 6 months PFS and ~ 15 months OS in advanced recurrent and metastatic HRAS mutant HNSCC patients Favorable safety and tolerability profile supports broad use in advanced patients as well as expansion to earlier therapeutic settings Fast Track Designation in HRAS Mutant HNSCC; potential for accelerated approval Novel mechanism and well tolerated profile could enable use in combination with standard of care, including immune therapy, targeted therapies and chemo Issued and pending patents provide exclusivity to 2036 in major markets

Cell membrane HRAS HRAS FTase HRAS FTase Tipifarnib HRAS (mutant) HRAS (wild-type) Tipifarnib inhibits farnesylation and signaling activity of the HRAS oncoprotein Farnesylation is essential for HRAS signal transduction activity HRAS mutations drive proliferation and resistance mechanisms in solid tumors Incidence of HRAS mutations in HNSCC is approximately 4-8% and varies by region Tipifarnib Inhibits Farnesylation – An Essential Modification Required for HRAS Activity

Source: Kura internal data Tipifarnib Tipifarnib Vehicle Vehicle Change in tumor volume (%)* HRAS wild type HRAS-mutant * Capped at 200%. Actual values 208-597% MAPK signaling Cell cycle arrest Apoptosis Angiogenesis Squamous differentiation Antitumor activity in PDX models HRAS membrane displacement pERK Ki67 c.CSP3 CD31 KRT4 Tipifarnib Displays Robust, Selective Activity in HRAS Mutant HNSCC Models

Ho et al. AACR-NCI-EORTC 2019 #384 (preliminary data as of 10/17/19) Efficacy-evaluable patients with HRAS mutant variant allele frequency (VAF) ≥ 20% and serum albumin ≥ 3.5 g/dL, or HRAS VAF ≥ 35% One patient treated off-protocol through compassionate use RUN-HN: Phase 2 Trial in HRAS Mutant HNSCC Time on Treatment ORR ~ 50% N=18 evaluable patients Not yet efficacy evaluable PR SD Not efficacy evaluable unconfirmed PR (uPR) 600 mg starting dose * Treatment Cycles (28 days) Durable Anti-Tumor Activity with Tipifarnib as a Monotherapy in Patients with HRAS Mutant HNSCC

Ho et al. ASCO 2020 #6504 (preliminary data as of 9/30/19) Efficacy-evaluable patients with HRAS mutant variant allele frequency (VAF) ≥ 20% and serum albumin ≥ 3.5 g/dL, or HRAS VAF ≥ 35% One patient treated off-protocol through compassionate use Progression-Free Survival with Tipifarnib and Last Prior Therapy in Patients with HRAS Mutant HNSCC Median PFS (months) 95% CI Lower Upper Tipifarnib HNSCC with high VAF, including additional patient (N=18) 5.9 3.5 19.2 Last prior line of therapy (n=17) 2.8 1.1 5.2 RUN-HN: Phase 2 Trial in HRAS Mutant HNSCC

Overall Survival in Patients with HRAS Mutant HNSCC Median OS (months) 95% CI Lower Upper HNSCC with high VAF, including additional patient (N=18) 15.4 7.0 46.4 Ho et al. ASCO 2020 #6504 (preliminary exploratory data as of 9/30/19) Efficacy-evaluable patients with HRAS mutant variant allele frequency (VAF) ≥ 20% and serum albumin ≥ 3.5 g/dL, or HRAS VAF ≥ 35% One patient treated off-protocol through compassionate use RUN-HN: Phase 2 Trial in HRAS Mutant HNSCC

* Feedback from end-of-Phase 2 meeting with FDA 2018 AIM-HN: Registration-directed trial of tipifarnib in HRAS mutant HNSCC Recurrent or metastatic patients after one prior line of platinum therapy Now open in ~90 clinical sites in the U.S., Europe and Asia Amended trial to enroll all HRAS mutant HNSCC patients regardless of variant allele frequency Intended to support an NDA seeking accelerated approval* SEQ-HN: Prospective observational cohort of HNSCC Matched case-control study designed to: Characterize natural history of HRAS mutant HNSCC patients and their outcomes after first line therapy Enable identification of patients for potential enrollment into AIM-HN May support potential FDA labelling discussions, post-approval commitments and commercial considerations Registration Strategy in HRAS Mutant HNSCC

Only ~1/3 of patients with advanced diagnosis survive 5 years4 SURVIVORS DO NOT SURVIVE 0 20 40 60 80 100 Outcomes with currently available therapies (including I-O therapy) are poor5 1 Bray et al. CA Cancer J Clin. 2018;68(6):394-424 2 Cramer et al. Nat Rev Clin Oncol. 2019 Nov;16(11):669-683 | ACS Cancer Facts and Figures 2020 3 Siegel et al. CA Cancer J Clin. 2020;70(1):7-30 4 National Cancer Institute. Introduction to head & neck cancer. https://training.seer.cancer.gov/head-neck/intro/. Accessed March 4, 2019 5 N Engl J Med. 2008 Sep 11;359(11):1116-27 | Keytruda & Opdivo package inserts | J Clin Oncol. 2007 Jun 1;25(16):2171-7 | J Clin Oncol. 2012 30:15_suppl, 5574-5574 Tipifarnib Has the Potential to be the First Small Molecule Targeted Therapy for HNSCC Patients OS First line: 10-15 mo Second line: 5-8 mo PFS First line: 3-5 mo Second line: 2-3 mo ORR First line: 20-36% Second line: 13-16% Head and neck squamous cell carcinoma ranks as the 7th leading cancer worldwide3 Globally, ~885,000 people develop head and neck cancer annually and ~450,000 die of HNSCC each year1 60,000+ cases of HNSCC per year in the U.S.2

Expansion Opportunities for Tipifarnib in HRAS and pi3kα dependent HNSCC

HRAS Dependent Tumors Represent a Significant Subset of HNSCC with Distinct Biology Several independent studies cluster HRAS mutant HNSCCs as part of a larger subset1 TCGA cohort shows overexpression of HRAS gene in 25-30% of HNSCC2 Average HRAS expression in HNSCC is 5-10x higher than in other tumor types Together with HRAS mutant tumors, HRAS-overexpressing HNSCC may represent a significant subset of HRAS dependent tumors with distinct biology that is targeted by tipifarnib HRAS Overexpressed 1 Campbell et al. (2018), Cell Rep. 23:194; Su et al. (2017), Theranostics, 7:1088; 2 International Cancer Genome Consortium (2013), Nat. Commun.| 4:2873
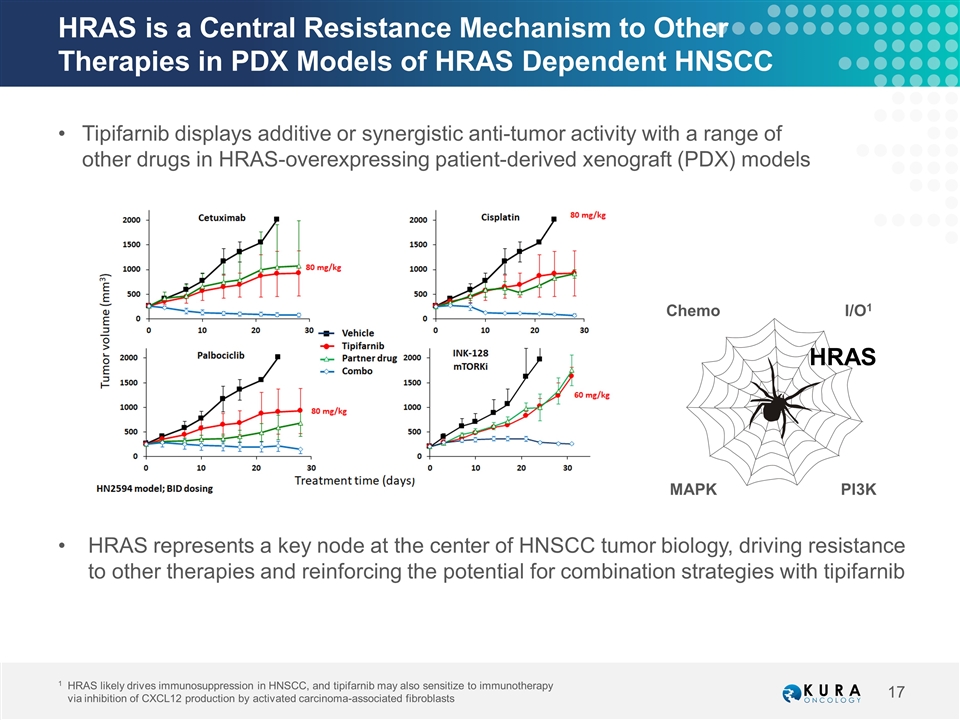
HRAS is a Central Resistance Mechanism to Other Therapies in PDX Models of HRAS Dependent HNSCC Tipifarnib displays additive or synergistic anti-tumor activity with a range of other drugs in HRAS-overexpressing patient-derived xenograft (PDX) models HRAS represents a key node at the center of HNSCC tumor biology, driving resistance to other therapies and reinforcing the potential for combination strategies with tipifarnib 1HRAS likely drives immunosuppression in HNSCC, and tipifarnib may also sensitize to immunotherapy via inhibition of CXCL12 production by activated carcinoma-associated fibroblasts HRAS PI3K I/O1 Chemo MAPK

Combinations of Tipifarnib and PI3Kα Inhibitor Demonstrate Robust Activity in HNSCC PDX Models Malik et al. EORTC-NCI-AACR 2020 #159 Tipifarnib used at reduced dose to simulate potential lower doses in combination (80à60mg/kg BID) BYL-719 used at reduced dose to simulate potential lower doses in combination (60à40mg/kg QD) Mean Tumor Volume ± SEM Days of treatment HRAS-mutant PIK3CA-mutant Wild-Type

Combinations of Tipifarnib and PI3Kα inhibitors Have Broad Therapeutic Potential in HNSCC HRAS-MAPK and PI3K-mTOR are complementary pathways in HNSCC Overexpression of WT HRAS reported to induce resistance to PI3Kα inhibition HRAS is reported to preferentially activate PI3K (vs. KRAS; vs. MAPK) HRAS mutation/over expression and PIK3CA mutations/amplifications account for 25-50% of HNSCC1 PIK3CA mutations/amplification: 30-40% (25% estimated overlap with HRAS overexpressing tumors) HRAS overexpression: 20-30% HRAS mutations: 4-8% (83% overexpress HRAS) Preclinical data is supportive of the combination; enhanced activity observed in both HRAS mutant/overexpressed and PIK3CA mutant/amplified populations of HNSCC 1TCGA Data References: Yan J et al (1998) JBC 273:24052 ; Gupta S et al (2007) Cell 129:957 ; Zhao L et al (2008) PNAS 105:2652

Patents cover combinations of tipifarnib with other agents (e.g., I/O) Additional patents possible with specific agents, doses, schedules, etc. Multiple issued U.S. patents covering biomarker-guided indications provide patent exclusivity to 2036 Additional patent applications pending in the U.S. and foreign countries for tipifarnib in other biomarkers and disease indications U.S. patents cover use of “any farnesyl transferase inhibitor” Researching FTIs with superior properties to tipifarnib Expect composition of matter IP on new discoveries Tipifarnib / FTI Patent Exclusivity Proprietary Biomarkers and Methods Combinations Novel FTI Program Layered patent strategy provides patent exclusivity to 2036 in major markets

KO-539: Menin Inhibitor in acute leukemias

KO-539: Menin Inhibitor Potent, selective, reversible, oral inhibitor of menin-KMT2A(MLL) protein-protein interaction for treatment of AML Novel MOA targeting epigenetic dysregulation leading to differentiation block and anti-tumor activity in 35% or more of AML Preliminary data from KOMET-001 Phase 1/2A dose-escalation study show encouraging safety, tolerability and clinical activity in multiple genetically defined subgroups of AML Focused monotherapy development strategy in multiple genetic subtypes: KMT2A(MLL) rearranged (5-10% of AML) NPM1 mutant (30% of AML) Other genetic subtypes (e.g., SETD2/RUNX1-mutant AML) Potential to combine with other targeted therapies and induction chemotherapy Issued and pending COM patents provide worldwide coverage to 2036

Targeting Menin-KMT2A(MLL) Interaction Provides Potential Therapeutic Intervention into Two Genetic Subsets of AML Targeting the menin-KMT2A(MLL) interaction to reverse epigenetic dysregulation in MLL-rearranged AML A central role for menin-KMT2A(MLL) interaction in epigenetic dysregulation in NPM1-mutant AML Kühn MW, et al. Cancer Discov. 2016;6(10):1166-1181 | Thorsteinsdottir U, et al. Mol Cell Biol. 2001;21(1):224-234 Patel SS, et al. Curr Hematol Malig Rep. 2020;15(4):350-359 | Brunetti L, et al. Cancer Cell. 2018;34(3):499-512

Burrows et al. AACR-NCI-EORTC 2017 LB-A27 100% (10/10) of animals treated with single-agent KO-539 cleared their leukemia and became long-term survivors Tumor growth inhibition was durable – no leukemia was detectable in blood or bone marrow two months after cessation of dosing KO-539 was well tolerated at tested dose levels Comparator compound (FLT3 inhibitor) was initially active, but all animals eventually relapsed AM7577 Overall Survival CD45+ Human AML Blasts Tolerability Last dose Last dose KO-539 Produces Lasting Complete Remissions in a NPM1 / DNMT3A / IDH2 / FLT3-Mutant AML Model

KOMET-001: Phase 1/2A First-in-Human Study of KO-539 in Patients with Relapsed or Refractory AML KOMET: Kura Oncology Menin-MLL Trial MLL/KMT2A-rearranged AML Cohort NPM1-mutant AML Cohort Determine recommended Phase 2 dose and/or MTD Safety and tolerability Pharmacokinetics Early evidence of antitumor activity Safety and tolerability Antitumor activity Phase 1 Dose Escalation Phase 2 Expansion Cohorts OBJECTIVES Accelerated, Adaptive Design

Continuous Daily Dosing of KO-539 Has Been Well-Tolerated with a Manageable Safety Profile No dose discontinuations due to treatment-related adverse events (AEs) No evidence of QT prolongation or other clinically significant ECG changes Treatment-related AEs (N=12) Grade ≥ 3 (all) Grade 1,2 (≥ 10%) Pancreatitis 1 (8.3%) 0% Lipase increased 1 (8.3%) 0% Neutrophil count decreased 1 (8.3%) 0% Tumor lysis syndrome 1 (8.3%) 0% Deep vein thrombosis 1 (8.3%) 0% Nausea 0% 3 (25%) Rash 0% 2 (16.7%) Diarrhea 0% 2 (16.7%) Wang et al. ASH 2020 #115 (preliminary data as of November 2, 2020)
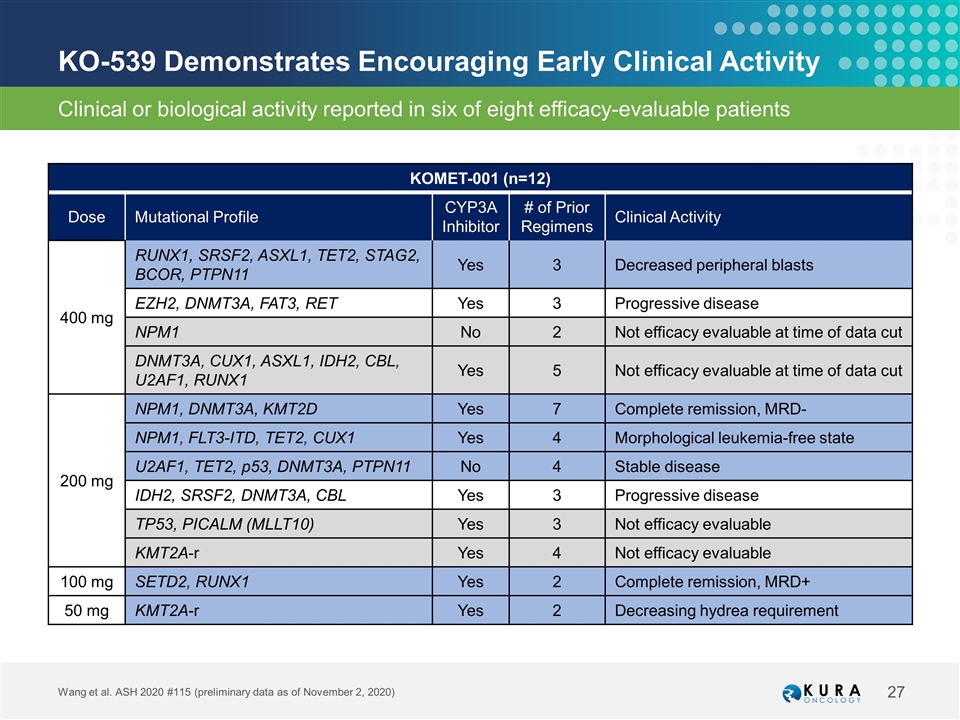
KO-539 Demonstrates Encouraging Early Clinical Activity Wang et al. ASH 2020 #115 (preliminary data as of November 2, 2020) KOMET-001 (n=12) Dose Mutational Profile CYP3A Inhibitor # of Prior Regimens Clinical Activity 400 mg RUNX1, SRSF2, ASXL1, TET2, STAG2, BCOR, PTPN11 Yes 3 Decreased peripheral blasts EZH2, DNMT3A, FAT3, RET Yes 3 Progressive disease NPM1 No 2 Not efficacy evaluable at time of data cut DNMT3A, CUX1, ASXL1, IDH2, CBL, U2AF1, RUNX1 Yes 5 Not efficacy evaluable at time of data cut 200 mg NPM1, DNMT3A, KMT2D Yes 7 Complete remission, MRD- NPM1, FLT3-ITD, TET2, CUX1 Yes 4 Morphological leukemia-free state U2AF1, TET2, p53, DNMT3A, PTPN11 No 4 Stable disease IDH2, SRSF2, DNMT3A, CBL Yes 3 Progressive disease TP53, PICALM (MLLT10) Yes 3 Not efficacy evaluable KMT2A-r Yes 4 Not efficacy evaluable 100 mg SETD2, RUNX1 Yes 2 Complete remission, MRD+ 50 mg KMT2A-r Yes 2 Decreasing hydrea requirement Clinical or biological activity reported in six of eight efficacy-evaluable patients

Case Study – Patient #2 2 prior lines of therapy KO-539 ~ 5 months C1D28 SD C3D28 CR, MRD+ C6D28 PR C8D28 PD Patient Characteristics Demographics 69-year-old male Mutational profile SETD2, RUNX1 Prior lines of therapies 2 (decitabine; CD33/CD3 bispecific antibody) KO-539 dose 100 mg, escalated to 200 mg during cycle 7 # of KO-539 cycles 8 CYP3A4 inhibitor Yes (fluconazole) Baseline bone marrow blasts 56% Clinical activity Complete remission, MRD+ (0.8% blasts) Grade ≥3 TRAEs Gr. 3 deep vein thrombosis Preliminary data as of November 2, 2020 Case Study – SETD2, RUNX1 Mutant AML

Case Study – Patient #3 7 prior lines of therapy KO-539 3 years and ~ 7 months C1D28 CR, MRD- C3D28 CR, MRD- Patient Characteristics Demographics 44-year-old female Mutational profile NPM1, DNMT3A, KMT2D, FLT3-TKD Prior lines of therapies 7 (incl. decitabine+venetoclax, gilteritinib, itacitinib, fludarabine, bortezomib) KO-539 dose 200 mg # of KO-539 cycles 3+ (on treatment) CYP3A4 inhibitor Yes (posaconazole) Baseline bone marrow blasts 14% Clinical activity Complete remission, MRD- (0% blasts) Grade ≥3 TRAEs Gr. 4 lipase increased, Gr. 3 pancreatitis, Gr. 3 neutrophil count decreased Preliminary data as of November 2, 2020 Case Study – NPM1, DNMT3A, KMT2D, FLT3-TKD Mutant AML
Summary of Preliminary Data from KOMET-001 KO-539 is a potent and selective inhibitor of the menin-KMT2A/MLL complex KO-539 has been well tolerated with a manageable safety profile to date Observed toxicities appear to be reversible and manageable No evidence of QTc prolongation KO-539 demonstrates encouraging signs of clinical activity in multiple genetically defined subgroups of AML KO-539 pharmacokinetics and clinical activity do not appear to be affected by co-administration of a CYP3A4 inhibitor Continuing to enroll patients in dose escalation, currently evaluating 600 mg cohort Anticipate determination of recommended Phase 2 dose in Q1 2021

Multiple Expansion Opportunities in Acute Leukemias Maximum Tolerated Dose and/or Recommended Phase 2 Dose Anticipated Q1 2021 Expansion Cohorts: 1) KMT2A(MLL)-r AML and 2) NPM1-mutant AML Potential 3rd AML Expansion Cohort: Other Genetics Pediatric Development Earlier Lines of Therapy in AML Combination with Standards of Care Other Disease Types, including Acute Lymphocytic Leukemia (ALL)

Relapsed/Refractory AML is a Challenging Disease Associated with Poor Outcomes Estimated 1,000-2,000 new cases in the U.S. per year7 (5-10% of AML) NCCN guidelines denote that MLL-r confers poor prognosis9 Estimated 6,000 new cases in the U.S. per year7 (~30% of AML) Known co-mutations confer worse prognosis8 and represent rational combination approaches KMT2A(MLL)-Rearranged AML NPM1-Mutant AML 1 Roboz et al. J Clin Oncol. 2014 Jun 20;32(18):1919-26 2 Stein et al. Blood. 2017 Aug 10;130(6):722-731 3 DiNardo et al. N Engl J Med. 2018 Jun 21;378(25):2386-2398 4 Taksin et al. Leukemia. 2007 Jan;21(1):66-71 5 Perl et al. Engl J Med. 2019 Oct 31;381(18):1728-1740 6 Cortes et al. Lancet Oncol. 2019 Jul;20(7):984-997 7 SEER statistics for AML in the US, accessed April 2020 8 Döhner et al. Blood. 2017 Jan 26;129(4):424-447 9 NCCN. AML Guidelines (version 3.2020). Accessed May 2020 Chemotherapy1 Drug Name AML Subset ORR Median OS Enasidenib IDH2 mutant 40.3% 9.3 mos2 Ivosidenib IDH1 mutant 41.6% 8.8 mos3 GO CD33+ AML 26% 11.6 mos4 Gilteritinib FLT3 mutant 34% 9.3 mos5 Quizartinib FLT3-ITD mut 27% 6.2 mos6 < 12 mos Targeted Therapies Credit: Dr. Wang, Roswell Park Comprehensive Cancer Center

*Cash, cash equivalents and short-term investments as of September 30, 2020 Targeted Oncology Advancing two wholly owned, targeted oncology drug candidates using a precision medicine approach; fast-to-market strategy Proprietary Pipeline Tipifarnib: Farnesyl transferase inhibitor Registration-directed trial in HRAS mutant head and neck squamous cell carcinoma (HNSCC) ongoing Opportunity to expand to HRAS and PI3Kα dependent tumors Multiple clinical proof-of-concept studies support significant lifecycle expansion opportunities KO-539: Menin inhibitor Potent and selective inhibitor of the menin-KMT2A(MLL) protein-protein interaction Potential to target ~35% of acute myeloid leukemia (AML) Preliminary Phase 1 data show encouraging safety, tolerability and clinical activity in multiple genetically defined subgroups of AML Strong Financials $325.4 million in cash* provides runway into 2023 Investment Highlights

DEVELOPING PRECISION MEDICINES FOR THE TREATMENT OF CANCER Corporate Presentation – December 2020
