l
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): January 13, 2020
KURA ONCOLOGY, INC.
(Exact name of Registrant as Specified in Its Charter)
|
Delaware |
001-37620 |
61-1547851 |
|
(State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
|
|
|
|
|
3033 Science Park Road, Suite 220, San Diego, CA
|
92121 |
|
|
(Address of Principal Executive Offices)
|
(Zip Code) |
|
Registrant’s Telephone Number, Including Area Code: (858) 500-8800
N/A
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instructions A.2. below):
|
☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
|
☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
|
☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
|
☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
|
Common Stock, par value $0.0001 per share |
KURA |
The Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 or Rule 12b-2 of the Securities Exchange Act of 1934.
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
Beginning on January 13, 2020, members of the management team of Kura Oncology, Inc. (the “Company”) will be providing presentation materials (the “Presentation”) to certain interested parties. A copy of the Presentation is attached hereto as Exhibit 99.1 and incorporated herein by reference.
The information contained in this Current Report on Form 8-K and in the accompanying Exhibit 99.1 are being furnished and shall not be deemed to be “filed” for the purposes of Section 18 of the Securities and Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that section and will not be incorporated by reference into any registration statement filed by the Company, under the Securities Act of 1933, as amended, unless specifically identified as being incorporated therein by reference. This Current Report on Form 8-K will not be deemed an admission as to the materiality of any information in this Current Report on Form 8-K that is being disclosed pursuant to Regulation FD.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits.
|
Exhibit Number |
|
Description |
|
99.1 |
|
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
KURA ONCOLOGY, INC. |
|
|
|
|
|
|
|
Date: January 13, 2020 |
|
By: |
/s/ James Basta |
|
|
|
|
James Basta |
|
|
|
|
Chief Legal Officer |

Developing Precision Medicines for the Treatment of Cancer J.P. Morgan Healthcare Conference January 13-16, 2020 Exhibit 99.1

Forward-Looking Statements This presentation contains forward-looking statements. Such statements include, but are not limited to, statements regarding our research, preclinical and clinical development activities, plans and projected timelines for tipifarnib, KO-947 and KO-539, plans regarding regulatory filings, our expectations regarding the relative benefits of our product candidates versus competitive therapies, and our expectations regarding the therapeutic and commercial potential of our product candidates. The words “aim,” “target,” “next steps,” “would,” “opportunity,” “expected,” “believe,” “may,” “will,” “estimate,” “promise,” “plan”, “continue,” “anticipate,” “intend,” “expect,” “potential” and similar expressions (including the negative thereof), are intended to identify forward-looking statements. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Risks that contribute to the uncertain nature of the forward-looking statements include: our preclinical studies and clinical trials may not be successful; the U.S. Food and Drug Administration (FDA) may not agree with our interpretation of the data from clinical trials of our product candidates; we may decide, or the FDA may require us, to conduct additional clinical trials or to modify our ongoing clinical trials; we may experience delays in the commencement, enrollment, completion or analysis of clinical testing for our product candidates, or significant issues regarding the adequacy of our clinical trial designs or the execution of our clinical trials may arise, which could result in increased costs and delays, or limit our ability to obtain regulatory approval; our product candidates may not receive regulatory approval or be successfully commercialized; unexpected adverse side effects or inadequate therapeutic efficacy of our product candidates could delay or prevent regulatory approval or commercialization; and we may not be able to obtain additional financing. Additional risks and uncertainties may emerge from time to time, and it is not possible for Kura’s management to predict all risk factors and uncertainties. All forward-looking statements contained in this presentation speak only as of the date on which they were made. Other risks and uncertainties affecting us are described more fully in our filings with the Securities and Exchange Commission. We undertake no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made.
* Includes cash, cash equivalents and short-term investments Investment Highlights Targeted Oncology Advancing pipeline of targeted drug candidates for selected solid tumors and hematologic malignancies Utilizing precision medicine approach; Fast-to-market strategy Proprietary Pipeline Tipifarnib: Farnesyl transferase inhibitor Clinical proof of concept achieved in HRAS mutant solid tumors and CXCL12-dependent hematology malignancies 1st Phase 2 registration-directed trial ongoing; 2nd planned in 2020 Significant lifecycle expansion opportunities in solid and liquid tumors KO-947: ERK inhibitor; Completing Phase 1 dose-escalation trial KO-539: Inhibitor of menin-MLL interaction; First patient dosed in September 2019 Near-Term Milestones Multiple anticipated development milestones across the pipeline in 2020 Financials Approximately $250 million in cash as of September 30, 2019*

Proven oncology drug discovery, development and commercialization expertise Kura Leadership Team and Board of Directors Leadership Team Troy Wilson, Ph.D., J.D. President and Chief Executive Officer Antonio Gualberto, M.D., Ph.D. Head of Development and Chief Medical Officer Marc Grasso, M.D. Chief Financial Officer and Chief Business Officer Kathleen Ford Chief Operating Officer James Basta, J.D. Chief Legal Officer Kirsten Flowers Chief Commercial Officer Board of Directors Faheem Hasnain Robert Hoffman Thomas Malley Diane Parks Steven Stein, M.D. Mary Szela Troy Wilson, Ph.D., J.D.

* Investigator-sponsored trials Product Candidate Pipeline Program Preclinical Phase 1 Phase 2 Pivotal Tipifarnib Farnesyl Transferase Inhibitor HRAS Mutant Indications CXCL12 Pathway Indications KO-947 ERK Inhibitor KO-539 Menin-MLL Inhibitor Head and Neck Squamous Carcinoma Urothelial Carcinoma* Lung Squamous Cell Carcinoma* Other Squamous Cell Carcinomas Angioimmunoblastic T-Cell Lymphoma CXCL12+ Peripheral T-Cell Lymphoma Chronic Myelomonocytic / Acute Myeloid Leukemias Pancreatic Cancer Solid Tumors AML Proof of concept achieved

01 Tipifarnib (Farnesyl Transferase Inhibitor) 02 03 KO-539 (Menin-MLL Inhibitor) KO-947 (ERK Inhibitor)

Farnesyl Transferase Inhibitors (FTIs): A Leadership Opportunity in Targeted Oncology Lifecycle expansion opportunities with validated biomarkers in multiple solid and liquid tumors Kura is building an exclusive position for the research, development and commercialization of tipifarnib and FTIs Accelerated approval paths with single-arm, ORR-driven studies in relapsed/refractory solid and liquid tumors Issued and pending patents provide exclusivity for FTIs in major markets

Potent, selective inhibitor of farnesyl transferase1 Well characterized with > 5,000 patients treated Durable responses previously reported in selected study patients, but appropriate genetic biomarkers were not identified at that time Kura scientists discovered proprietary biomarkers (HRAS and CXCL12 pathway); validated in five Phase 2 proof-of-concept studies Manageable safety profile observed as monotherapy (< 25% treatment discontinuation) Tipifarnib adverse events2: Myelosuppression (neutropenia 25%, anemia 31%, thrombocytopenia 19%) Non-heme > 25%: fatigue (41%) and GI unspecific (nausea 47%, anorexia 33%, diarrhea 32%, vomiting 32%) Tipifarnib: A First-in-Class Farnesyl Transferase Inhibitor for the Treatment of Cancer 1 End et al. 2001. Cancer Res. 61:131‑37 2 Adverse events reported from 472 solid tumor patients Kura has unlocked the potential of tipifarnib and FTIs as targeted therapeutics in oncology

CXCL12 and its receptors (CXCR4, CXCR7) link cancer cells to the tumor microenvironment CXCL12 pathway activation drives cancer phenotype; poor prognosis Tipifarnib inhibits farnesylation of key regulatory proteins involved in CXCL12 production HRAS mediates signal transduction and growth and proliferation of tumor cells HRAS mutations drive resistance to SOC therapies; poor prognosis Tipifarnib inhibits farnesylation and signaling activity of the HRAS oncoprotein HRAS Mutant Solid Tumors CXCL12-Dependent Tumors FTIs Inhibit Two Distinct Critical Pathways and Drive Activity in Biomarker-Defined Tumors

1 Ho et al. AACR-NCI-EORTC #384 (preliminary data as of 10/17/19) 2 Feedback from end-of-Phase 2 meeting with FDA Registration Strategy in HRAS Mutant HNSCC: Potential for Accelerated Approval HNSCC represents a significant unmet need as standard of care (SOC) provides limited clinical benefit in ORR (~13-16%) and PFS (~2 months) in 2nd line patients HRAS mutations are a negative prognostic factor and primary mechanism of resistance to SOC In RUN-HN study, tipifarnib compared favorably to patients’ prior line of therapy ORR (56% vs. 0%) Median PFS (6.1 vs. 2.8 months) RUN-HN: HRAS Mutant Head & Neck Squamous Cell Carcinomas1 AIM-HN: Registration-Directed Trial of Tipifarnib in HRAS Mutant HNSCC ORR: 56% Median PFS: 6.1 months At least 59 evaluable recurrent or metastatic patients after platinum therapy Trial initiated in November 2018; Full enrollment planned by Q1 2021 Intended to support an NDA seeking accelerated approval2

Dr. Se Hoon Park, Samsung Medical Center (unpublished data) Proof-of-Concept in Urothelial Carcinoma Demonstrates Potential for Label Expansion HRAS Mutant Urothelial Carcinoma Potential for Label Expansion in HRAS Mutant Tumors HNSCC Earlier lines of therapy: 1st line combination with SOC I/O and adjuvant setting Broaden patient pool in low HRAS mutant variant allele frequency in combination Urothelial carcinoma Lung squamous cell carcinoma Other SCCs Penile Vulvar Cutaneous ORR: 33% Median PFS: 5.1 months

CXCL12 and its receptors (CXCR4, CXCR7) link cancer cells to the tumor microenvironment CXCL12 pathway activation drives cancer phenotype; poor prognosis Tipifarnib inhibits farnesylation of key regulatory proteins involved in CXCL12 production HRAS mediates signal transduction and growth and proliferation of tumor cells HRAS mutations drive resistance to SOC therapies; poor prognosis Tipifarnib inhibits farnesylation and signaling activity of the HRAS oncoprotein HRAS Mutant Solid Tumors CXCL12-Dependent Tumors FTIs Inhibit Two Distinct Critical Pathways and Drive Activity in Biomarker-Defined Tumors

Witzig et al. ASH 2019 #468 (preliminary data as of 11/11/19) Registration Strategy in AITL: Single-Arm ORR Trial Provides Potential for Accelerated Approval ORR (PPS): 50% Median DOR: 6.6 months Phase 2 Study in Angioimmunoblastic T-Cell Lymphomas AITL represents a significant unmet need as SOC provides limited clinical benefit (~25% ORR; 2-3 months PFS) CXCL12 is a negative prognostic factor for PTCL/AITL AITL is characterized by high CXCL12 expression; histology serves as surrogate for CDx Identified AITL molecular subset with improved overall response rate (70%); KIR3DL2 mutation increases dependency on CXCL12 Registration-Directed Trial of Tipifarnib in AITL and AITL-like histologies At least 128 patients R/R to at least one prior systemic cytotoxic therapy Two independent primary objectives: 1) ORR in AITL and 2) ORR in AITL molecular subset (determined retrospectively) Plan to initiate in 2H 2020, intended to support NDA seeking accelerated approval*
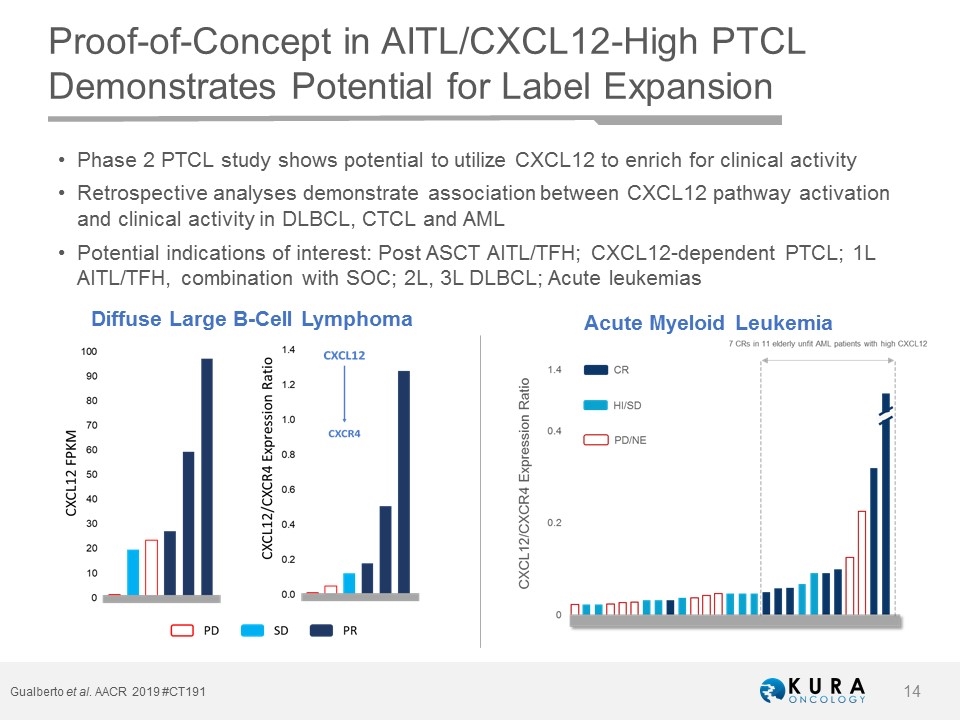
Gualberto et al. AACR 2019 #CT191 Proof-of-Concept in AITL/CXCL12-High PTCL Demonstrates Potential for Label Expansion Diffuse Large B-Cell Lymphoma Phase 2 PTCL study shows potential to utilize CXCL12 to enrich for clinical activity Retrospective analyses demonstrate association between CXCL12 pathway activation and clinical activity in DLBCL, CTCL and AML Potential indications of interest: Post ASCT AITL/TFH; CXCL12-dependent PTCL; 1L AITL/TFH, combination with SOC; 2L, 3L DLBCL; Acute leukemias Acute Myeloid Leukemia

Potential for Label Expansion in CXCL12-Dependent Solid Tumors: Pancreatic Cancer 1 Samarendra, et al. Br J Cancer. 2017;117:124–15 2 Gualberto et al. JCO 2019. 37, suppl: 275 (gene expression data from TCGA) 3 Kura Oncology, data on file Rationale High CXCL12 expression is associated with reduced overall survival in patients with pancreatic cancer1 CXCL12 expression and KRAS mutant allele frequency (MAF) are mutually exclusive: ~30% of PDCA carry <5% KRAS MAF and express high levels of CXCL122 Tipifarnib downregulates CXCL12 secretion from pancreatic stellate cells and inhibits the growth of high CXCL12, low KRAS mutant PDCA xenografts3 OS benefit (10.2 vs. 5.9 months, HR=0.52, p<0.0001) observed with tipifarnib treatment in patients with CXCL12-expressing PDCA tumors (identified by clinical characteristics) in retrospective analyses2 Next Steps Anticipate initiating a Phase 2 POC study in 2H 2020

Indication Patients* Angioimmunoblastic T-cell lymphoma (AITL) & AITL-like histologies*** 1,300+ Acute myeloid leukemia (AML) 6,400+ Diffuse large B-cell lymphoma (DLBCL) 9,000+ Pancreatic 17,000+ Indication Patients* Head & neck squamous cell carcinomas (HNSCC)** 2,900+ Urothelial carcinomas 4,600+ Lung squamous cell carcinomas Other squamous cell carcinomas Tipifarnib: Broad Potential Market Expansion Opportunity *Estimates of the biomarker-positive subsets across all lines of therapy **HNSCC population with HRAS variant allele frequency ≥ 20% (TCGA) ***Does not include additional opportunities in peripheral T-cell lymphoma (PTCL) or cutaneous T-cell lymphoma (CTCL) HRAS Mutant Solid Tumors Populations Based on Annual U.S. Incidence CXCL12 Pathway Indications Populations Based on Annual U.S. Incidence Indication Expansion Treatment Setting Treatment Setting Indication Expansion

Tipifarnib / FTI Patent Exclusivity Patents cover combinations of tipifarnib with other agents (e.g., I/O) Additional patents possible with specific agents, doses, schedules, etc. Multiple issued U.S. patents covering biomarker-guided indications (e.g., HRAS mutant HNSCC, CXCL12-expressing PTCL) and provide patent exclusivity to 2036 and beyond Include claims to biomarker, dose, schedule and tumor Additional patent applications pending in the U.S. and foreign countries for tipifarnib in other biomarkers and disease indications U.S. patents cover use of “any farnesyl transferase inhibitor” Proprietary Biomarkers and Methods Combinations Researching FTIs with superior properties to tipifarnib Expect composition of matter IP on new discoveries Novel FTI Program Broadest claims cover any FTI, providing Kura an opportunity to have an exclusive leadership position for FTIs in oncology Layered patent strategy provides patent exclusivity to 2036 and beyond in major markets

KO-539 (Menin-MLL Inhibitor) 03 01 Tipifarnib (Farnesyl Transferase Inhibitor) 02 KO-947 (ERK Inhibitor)

Potent, selective small molecule inhibitor of ERK1/2 Demonstrates prolonged pathway modulation in preclinical tumor models Multiple tumors, including molecularly-defined squamous cell carcinomas and adenocarcinomas, identified as sensitive to KO-947 as monotherapy in preclinical models Mechanism-based and SOC combinations under evaluation Favorable pharmacology enables intermittent dosing schedules Potential biomarkers, including 11q13 amplifications in SCCs, have been identified for patient enrichment Phase 1 dose-escalation trial ongoing KO-947: Potent Inhibitor of ERK1/2 ERK RAS RAF MEK Nucleus KO-947

Burrows et al. AACR 2017 #5168/11 KO-947 Demonstrates Robust Single-Agent Activity in Preclinical Studies Broad profiling in ~ 200 patient-derived xenograft (PDX) models Consistent and compelling activity in diverse indications on intermittent schedules Robust activity in preclinical models of HNSCCs and ESCCs with 11q13 amplifications Leverages existing HNSCC clinical and diagnostic infrastructure for tipifarnib

First-in-human study in patients with advanced solid tumors Currently evaluating two dosing regimens, including once-weekly dosing and a more frequent intermittent schedule Dose-escalation objectives: Determine recommended Phase 2 dose (RP2D) and/or maximum tolerated dose (MTD) Investigate safety and tolerability Characterize pharmacokinetics Assess early evidence of anti-tumor activity Anticipate reaching RP2D and/or MTD with potential to enrich in patients with 11q13-amplified SCC in first half of 2020 KO-947: Phase 1 Clinical Trial

01 Tipifarnib (Farnesyl Transferase Inhibitor) 03 KO-539 (Menin-MLL Inhibitor) KO-947 (ERK Inhibitor) 02

1 N Engl J Med. 2012 Mar 22;366(12):1079-89 | Haematologica. 2007 Jun;92(6):744-52 | Anticancer Res. 2005 May-Jun;25(3B):1931-44 Potent, selective small molecule inhibitor of the menin-MLL protein-protein interaction Robust antitumor activity observed in mixed lineage leukemias rearranged (MLL-r) as well as disseminated NPM1 mutant and DNMT3A mutant AML PDX models Preliminary preclinical data suggests anti-leukemic activity by induction of myeloid differentiation in AML blasts NPM1, MLL-r and MLL-PTD mutations occur in ~40% of AML patients1 Granted Orphan Drug Designation for the treatment of AML in July 2019 Phase 1 dose-escalation trial ongoing KO-539: Potent Inhibitor of Menin-MLL Interaction The menin-MLL complex appears to be a central node in epigenetic dysregulation driven by several distinct oncogenic driver mutations important in diverse leukemias and myeloproliferative disorders

Burrows et al. AACR-NCI-EORTC 2017 LB-A27 KO-539 Produces Lasting Complete Remissions in a NPM1 / DNMT3A / IDH2 / FLT3-Mutant AML Model 100% (10/10) of animals treated with single-agent KO-539 cleared their leukemia and became long-term survivors Tumor growth inhibition was durable – no leukemia was detectable in blood or bone marrow two months after cessation of dosing KO-539 was well tolerated at tested dose levels Comparator compound was initially active, but all animals eventually relapsed AM7577 Overall Survival CD45+ Human AML Blasts Tolerability

First-in-human study in patients with relapsed/refractory AML First patient dosed in September 2019 Administered as a once daily oral dose in 28-continuous-day cycles Dose escalation objectives: Determine recommended Phase 2 dose and/or MTD Investigate safety and tolerability Characterize pharmacokinetics Assess early evidence of antitumor activity Anticipate reaching RP2D with potential to enrich in NPM1-mutant AML and MLL-rearranged genetically defined subgroups this year KO-539: Phase 1 Clinical Trial

* As of September 30, 2019 Forecasted Milestones & Financial Highlights Program Milestone Status Tipifarnib Farnesyl Transferase Inhibitor HRAS Mutant Indications Data from Phase 2 trial in urothelial carcinoma 2020 Potential for full enrollment in AIM-HN Q1 2021 CXCL12 Pathway Indications Data from Phase 2 trial in CMML 1H 2020 Initiation of registration-directed trial in AITL 2H 2020 Initiation of proof-of-concept study in pancreatic cancer 2H 2020 KO-947 ERK Inhibitor RP2D and/or MTD in Phase 1 trial with enrichment in 11q13-amplified SCC 1H 2020 KO-539 Menin-MLL Inhibitor RP2D in Phase 1 trial with enrichment in genetically defined subgroups 2020 Financial Highlights Nasdaq: KURA Shares outstanding: 45.3M basic, 4.1M options* Cash, cash equivalents and short-term investments: $250.1M*

Developing Precision Medicines for the Treatment of Cancer
